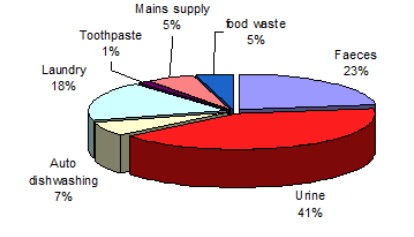
Phosphate in domestic sewage is comes mainly from urine, detergents and as an anti-corrosion additive to tap water.
All animals excrete the phosphate they do not need in their urine and this is accounts for reportedly (below) about two thirds of phosphate in sewage influent.
Phosphate is put into the detergent to soften the water and aid washing.
It does this by trapping the calcium in the water that is responsible for making it ‘hard’ which produces a scum during washing. It is also tne basis of surfactants used in washing; these are substances that make water 'wetter' and spread more easily over dishes and in clothes (technically they lower the surface tension of water). Surfactants are the chemicals that make water form lather and bubbles. These substances are present in most detergents and dishwasher tablets. Not all detergents have phosphates in them as there are many other suitable chemicals but the industry argues that phosphate is the best and cheapest. The relative phosphate loading due to each of these sources seems to be a matter from some debate; Wessex Water (2018) quotes 25% of total phosphate in sewage influent (below and right).
.
Phosphate is effective in stopping corrosion of cast iron pipes and, in particular, of lead pipes. Lead poisoning from lead pipesis long known and while most pipesare now plastic, this is still significant. Wessex water reports that this is the source of about 5% of phosphate in sewage influent. The concentration of phosphate in the Parish drining water is above 3ppm which, in a watercourse, would be classified by the Environment gency as bad, the lowest classification level.
|
| Phosphorus in sewage influent. From the 2018 submission by Wessex Water to the Drinking Water Inspectorate (DWI). (Link to the right) |
Phosphate mining, (like fossil fuel extraction and the fixing of atmospheric nitrogen) is an example of an unsustainable that is gradually overloading the biosphere.
Phosphate used in ferilisers and detergents is not synthesised chemically, it comes in raw deposits and is mined and refined. The main phosphate producing countries are China, Morocco, the United States, Kazakhstan, Brazil and other north Afican and middle eastern countries. The total world output is about 250 million tonnes. A major source of high quality phosphate used to be the Pacific Island of Nauru were 80% of the isaland was strip-mined leaving the island, inhabited for at least 3000 years, unable to support its population.
It is important to note that globally, the transfer of nutrient compounds such as phosphate and nitrate from inert deposits (in the case of nitrogen, the air) into active nutrients in the biosphere is ultimately unsustainable. There is much discussion now around the consequences of the biosphere gradually becoming overloaded with these nutrients such that they are no longer being fully recycled. It is a debate very similar to the unsustainable loading of the atmosphere by carbon dioxide of fossil fuel origin.
It is not necessary for detergents to contain phosphate. Ecover is probably the best-known phosphate free detergent-developed originally by a Belgian chemist in the seventies who became concerned, even then, with the unnecessary pollution from detergents.
A decade ago the European Parliament voted to ban phosphate in detergents but this was advisory only as it was ultimately a decision that was devolved to individual countries. The UK decided on a maximum of 0.5% for washing powders and 0.3% for dishwashers (subsequently rephrased as 0.5g per recommended amount used in the wash). For a village the size of Martock this means that probably around 40kg of phosphate from this source enters (and leaves) the sewage treatment plant each week.
Canada has banned the use of phosphate in detergents completely (following the pollution of lakes) and In the US, so many States have banned phosphate in detergents that the companies have apparently largely stopped making them with phosphate rather than coping with the inconsistent market.
Some phosphate is removed by sewage works but most is not as it is a soluble naturally occurring substance that is not filtered out by any of the normal sewage treatment processes .
Because phosphate is a 'limiting nutrient' it is present in quite small quantities in sewage effluent and removing such low concentrations is technically quite difficult and costly and tends to introduce other chemicals–such as aluminuum–into the water that are not particularly desirable. A few of the Wessex Water Sewage Treatment Plants (STWs) in the local catchment area remove phosphate; Yeovil and Ilchester, for example, which both discharge into the Yeo. Phosphate is not removed by any of the STWs that discharge into the Parrett. Addition of phosphate removal stages to Crewkerne, Meriott, Martock and South Pethertnn Sewage works are planned in the current workplan which ends in 2024. No reasons seem to have been given why this cannot happen earlier.
Martock Sewage Works serves a community of old buildings. It must therefore take storm water and domestic sewage together as this is often not separated in old buildings. Because of this Martock Sewage Works often overflows– on average about once a week–and raw sewage enters the River Parrett via a small tributary, Hinton Meads Brook. In 2019, for example, 58 spills were registered lasting in total 591 hours. In 2021, 56 spills lasted 522 hours. This is high compared with most other Sewage Treatment Works in the area and, given the relatively large population it serves, make Martock Sewage Works possibly one of the most polluting in the Levels catchment area. The extent to which such incidents exacerbate the effects of phosphate pollution is not apparently known.
In Victorian times, sewage works commonly used reed-beds downstream for the final treatment stage to remove soluble nutrients and this was, and is, remarkably effective at removing phosphate. The principle is simple; phosphate in solution is taken up to a variety of growing plants and then released in a controlled manner by the normal phosphate cycle (or the plant is harvested and the phosphate recycled). The key consequence is that no single plant, such as blanket alga, dominates the process. This method is still common worldwide–partly a relic of the Empire and of missionary activity–but is no longer common in the UK as it requires land. In Yorkshire, however, Fairburn Ings near Castleford, is now a significant and much visited RSPB reserve revived originally in the sixties to cope with all the detergent produced by the woollen industry and dumped in the river Aire. Sewage reedbeds everywhere are typically very biodiverse and are often very popular with birdwatchers.
It is clear that phosphate pollution is not something new and not something inherently difficult to deal with. A rule of thumb I have seen used (at a mission station on the Namibia/Angola border) in designing reed-beds is a minimum of about 1 m2 per person serviced so a hectare could probably be suffcient for Martock. This is, essentially, what is behind the offsetting proposal for the removal of phosphate in sensitive areas such as the Kent Stour and the Solent, as is, we understand, envisaged for the Somerset Levels. Its main, and obvious, drawback is that it that it will only be effective if the water entering the bed is actually significantly polluted and herein lies a problem; we only have data for the main rivers. |
 The River Parrett just downstream of Gawbridge. The bubbles indicate the two flap outflow of Martock STW which typically increases the phosphate concentration here by around 25%
The River Parrett just downstream of Gawbridge. The bubbles indicate the two flap outflow of Martock STW which typically increases the phosphate concentration here by around 25%